Methane drained from coals seams has been identified by the National Energy Board as the next new supply likely to augment the production of natural gas from conventional sources in Canada. Under the Climate Technology Initiative negotiated at the time of the Kyoto Protocol in late 1997, Canada is co-leader of an international project to evaluate the possibility of using carbon dioxide captured from utilities and other sources consuming fossil fuels as a flushing gas in CBM technology. Flue gas might be used directly in which case some nitrogen would be expected to appear in the product methane. Whether essentially pure CO2 or flue gases are employed, the carbon dioxide contained would be sequestered in coal seams which are not expected to be mined in the foreseeable future.In this paper the application of methane gas recovered from coal seams is examined in energy conversion technologies equipped for the capture of carbon dioxide in a relatively pure state although nothing precludes the direct use of flue gas with appropriate process modifications. Both separated and integrated energy conversion facilities are considered in this paper. This linkage is complicated by the fact that two moles of carbon dioxide are required to release one mole of methane from the coal. It is concluded that the integrated option is worthy of further study because such a combined process arrangement allows the flexible generation of electricity and the co-production of either hydrogen or liquid fuel useful for transportation applications (here taken as methanol) with low overall emissions of greenhouse gases.
At least two trials have been conducted in the U.S.A. using carbon dioxide obtained from natural sources as the flushing gas. Though the Canadian Gas Potential Committee has identified a large CBM resource located mainly in coal seams in Alberta,5 it is not obvious that Canada has significant, if any, comparative advantage in this field. Moreover, large untapped resources of conventional natural gas exist in the Middle East and possibly in some other locations around the world which could be delivered to coastal markets in the U.S.A. by tanker in liquefied form as LNG. Given that Canadian gas now serves several of these large markets, this supply of LNG may effectively place a cap on the price of natural gas at the wellhead in Canada. It is not known yet whether this capping price will be sufficiently high to allow the extensive production of CBM in Alberta which gas is inherently more costly to produce than that from conventional resources.
In this paper, the 2-for-1 sequestering capability of the CBM option is examined in the context of the generation of electricity and the production of hydrogen or methanol in processes equipped for the capture of essentially pure carbon dioxide. Flue gases containing nitrogen, surplus oxygen, water vapour, as well as the carbon dioxide might conceivably be applied directly for this purpose with possible cost savings given appropriate process modifications though there are limits on the acceptable level of oxygen and water vapour in the injection gas. This promising option is not considered further here. The hydrogen produced might be consumed in fuel cells in vehicles or for the generation of electricity at a smaller scale for local or household purposes remote from the site. The methanol is produced as a proxy for any liquid fuel useful for transportation applications. There are no inherent reasons why gasoline or other hydrocarbon liquids useful for transportation applications could not be synthesized from the CBM gas, if preferred.
In effect, the overall process arrangement would consist of the generation of electricity from any fossil fuel (probably coal in Alberta but natural gas is also a possibility) in facilities equipped to capture the carbon dioxide produced in combustion. This carbon dioxide would then be sequestered in the coal seam with the concurrent release of half as much methane on a molar basis. The methane so released could be used for several purposes including the generation of additional electricity but here it will be dedicated either to the production of hydrogen or methanol because of its high hydrogen content. Carbon dioxide would also be captured from the latter processes to augment the volume of flushing gas so as to increase the supply of displaced methane still further. Such process combinations have been studied by Williams6 with particular reference to the opportunities in China.

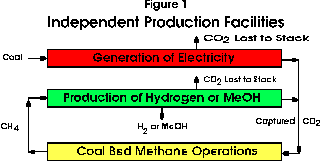
The separate generation and production facilities are illustrated in block format in Figure 1. Electricity is generated by the combustion of coal which is currently the main source of this form of energy in Alberta. The carbon dioxide is separated from either existing modified conventional generation facilities or from new technologies such as the emerging Integrated-Gasification Combined-Cycle (IGCC) process. The captured gas is pipelined to the site of the CBM operations. The methane released from the CBM stage is then used to produce hydrogen or methanol in a second facility. Carbon dioxide is also recovered from this second stage of processing and combined with that from the first source for return to CBM operations. There may also be an institutional advantage in this arrangement in that the two plants can be under separate ownership and control. If the production of methanol is desired, more of this liquid could be produced from the methane released from the CBM stages in the conventional process than in the integrated case to be discussed in detail below because of the more favourable hydrogen-to-carbon monoxide ratio in the process gases. It is also possible some of the captured carbon dioxide could be blended with methane to augment methanol production in the conventional process.
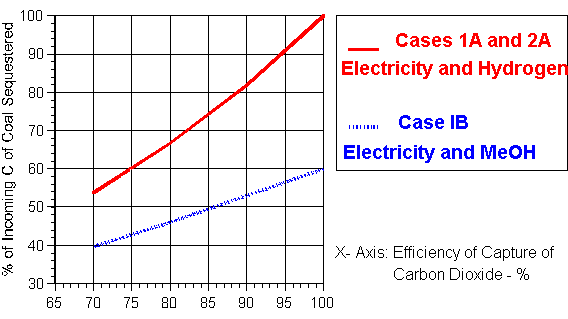
Case 1A deals with the generation of electricity and the production of hydrogen in separate facilities. For every mole of C in the CH4 released in the CBM process, 2.0 moles in the form of CO2 must be returned to the ground given the 2-for-1 rule. For the case when 80% of the carbon fed to the conventional hydrogen plant is captured for sequestering, then for each mole of CH4 reformed, 0.8 moles of CO2 are available which may be deducted from the 2.0 moles of CO2 needed to release one mole of CH4 in the CBM operations. This means that 2.0 - 0.8 = 1.2 moles of CO2 must be captured from the electrical generation stage. With 80% of the carbon dioxide in the combustion product also assumed captured in the generation stage, the incoming fossil fuel, usually coal, must contain 1.5 moles of C to maintain the carbon balance. The emissions to the atmosphere from the hydrogen plant and generation are then 0.2 and 0.3 moles respectively to total 0.5 moles CO2. Therefore, the overall percentage of the carbon from the fossil fuel feed released to the atmosphere will be 33.3%. Hence 66.7% of the C entering with the fossil fuel will be sequestered on an overall basis.
The results plotted in Figure 2 for Case 1A cover a range of capture from 70-100% and thus include the above 80% case. This rate is assumed the same for both the generation and hydrogen production stages. If the capture rate were 90% and 100% effective in the two process stages, 81.9% and 100% respectively of the C entering with the fossil fuel would be sequestered.
The relationship between the proportion of the carbon sequestered which enters the process with the coal approximates a straight line. Allowing for the methane needed for the underfiring of the reformers, the hydrogen production in a conventional facility will be of the order 2.6 moles per mole C in the CBM gas. The electrical generation will depend upon the efficiency of the facility employed.
Data is also plotted in Figure 2 for Case 1B for a range of capture efficiencies from 70-100%. If the capture rate were increased first to 90% and then to 100% in both process stages, then the percentage of the carbon entering with the fossil fuel sequestered would increase to 53.0% and 60.0% respectively when the carbon of the methanol produced is counted as an emission of carbon dioxide against its future use in vehicles. The proportion of the carbon of the fossil fuel sequestered is again approximately linear with the efficiency of capture of the carbon dioxide.
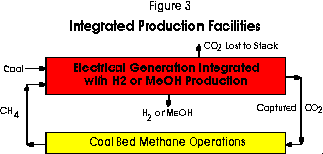
In the second block configuration illustrated in Figure 3, the process stages are integrated. This combination is possible in an integrated-gasification combined-cycle power plant (IGCC) equipped for the conversion of the carbon monoxide in the process gases to hydrogen with steam in the shifting process. The integrated arrangement allows certain process efficiencies as only one gas purification and one carbon dioxide separation stage are required. Charging the gasifier with the methane released in the CBM stage along with the coal increases the hydrogen content of the product stream which is an important advantage when hydrogen or methanol are to be produced. Moreover, the unit capital requirements for the expensive gasification stage is lowered because its output is usually limited by its coal and ash-handling capability, not by its gas carrying capacity.
Greater flexibility in operation is possible because hydrogen production can be curtailed during peak periods of electrical demand to permit greater generation. The oxygen normally required for gasification could also be produced off-peak. The hydrogen content of the coal adds to the production of this gas whereas in the independent facilities case, all this element appears as water vapour in the stack gas following combustion.
Hydrogen may be separated in membranes, by using the pressure swing adsorption processes, or alternatively, conventional physical absorption processes may be applied to separate the carbon dioxide leaving the hydrogen behind. The carbon dioxide captured would be sequestered in the CBM operations to release methane. An entrained-flow type reactor, such as employed in the Texaco Process, would be preferred for the simultaneous gasification of the two fuels because very little residual methane is wanted in the product gas stream. The fraction of hydrogen not separated for other purposes would be combusted with air in gas turbines to generate electricity and the exhaust gases would provide the energy for the heat recovery steam cycle in the normal combined-cycle process. There is uncertainty, however, whether a high-hydrogen fuel gas will prove satisfactory as a fuel in conventional gas turbines because of the low molecular weight of the combustion gas to be expanded in the turbine. Issues of this kind including the study of the separation processes are in the province of the Greenhouse Gas R and D Programme of the International Energy Agency to which Canada belongs.7
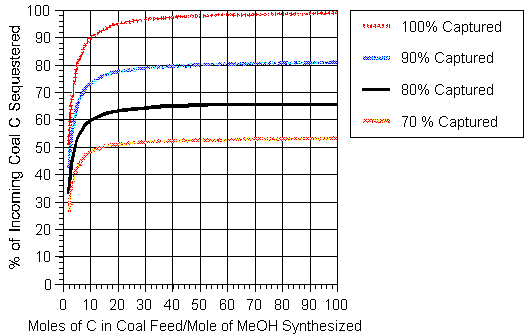
The results of the above calculation are illustrated in Figure 4 which covers the range of interest. The results are strongly sensitive to the ratio of the number of moles of carbon in the coal gasified to the moles of methanol produced below a value of 10. If the capture rate were increased to 90% and 100% for the case when x = 2, then the percentage of the carbon entering with the fossil fuel sequestered would be 40.9% and 50.0% respectively. If the capture rate were 90% when x = 100, the percentage sequestered would be 83.0% and with perfect capture, 99%. As in the case of hydrogen production, the synthesis of methanol could be curtailed during periods of peak electrical demand.
Nevertheless, the advantages of the integrated case seem large enough to warrant at least a preliminary process assessment. The synthesis of other hydrocarbon liquids in place of methanol could also be studied.
NOTE: A related paper Incorporating Carbon Dioxide Sequestration and Coal Bed Methane Recovery into Hydrogen Production from Coal - Economics and Environmental Aspects by Pamela Spath and Wade Ames of the National Renewable Energy Laboratory, Golden, Colorado, was published in a Symposium on Carbon Dioxide Utilization and Sequestration held by the American Chemical Society in Washington, D.C. 20-24 August 2000 (Vol. 45 No.4 of the ACS Division of Fuel Chemistry).
January 2000
Revised February 2000
19 Lambton Avenue, Ottawa, Ontario, K1M 0Z6
Tel: (613) 745-6279,
E-Mail: jhwalsh@ca.inter.net